Case Info
Oxbryta Sickle Cell Vaso-Occlusive Crisis Lawsuit
Two recent studies have shown that Oxbryta (voxelotor) may increase the risk for vaso-occlusive crisis, stroke, organ failure and death in people who took the medication used to treat Sickle Cell Disease (SCD). The U.S. Food and Drug Administration (FDA), along with pharmaceutical manufacturer Pfizer has announced a recall for all lots of Oxbryta.
Sickle Cell Disease patients who took Oxbryta and experienced vaso-occlusive crisis, stroke or organ failure or loved ones of those who died after taking the medication may be eligible for compensation.
Oxbryta Studies Show Increased Risk
Two studies mandated by the FDA, have determined that taking Oxbryta may increase the risk of a serious condition known as a vaso-occlusive crisis, which can cause organ failure, stroke, or death. The FDA has stated that the benefits of Oxbryta do not appear to outweigh the risks.
Healthcare practitioners have been advised to closely monitor patients who were prescribed Oxbryta and to report serious side effects to the FDA. Patients currently taking Oxbryta should contact their healthcare provider before discontinuing the medication.
Vaso-Occlusive Crisis
Studies have shown that Oxbryta may increase the risk of vaso-occlusive crisis when compared to placebo. The FDA has determined that the risks of taking Oxbryta may outweigh the benefits.
Symptoms of vaso-occlusive crisis may include:
- Fever
- Pain
- Swelling
- Kidney Failure
- Stroke
- Death
About Sickle Cell Disease
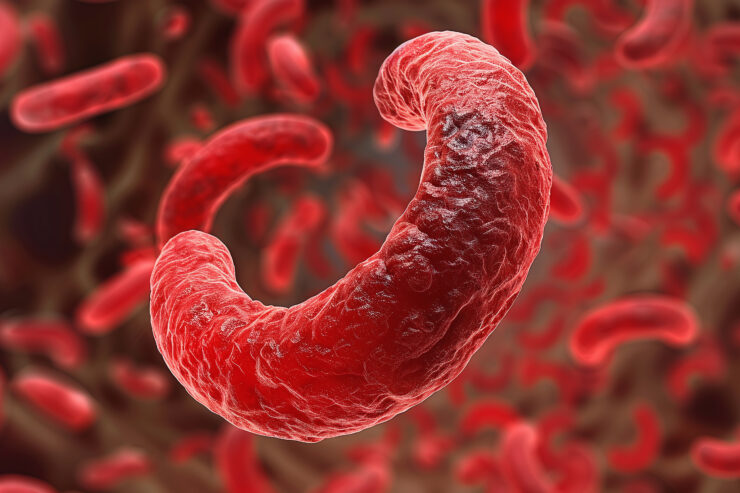
Sickle Cell Disease or Sickle Cell Anemia is an inherited disorder which affects hemoglobin, a protein necessary to carry oxygen in red blood cells.
Normally, red blood cells are flexible, donut-shaped cells which slide easily through the blood vessels, transporting oxygen to tissues. Sickle cell disease causes blood cells to become hard and sticky c-shaped or “sickle-shaped” cells which become stuck in blood vessels. It may also cause red blood cells to die early, requiring the body to work harder than normal to replenish levels.
Sickle Cell Disease is also sometimes referred to as Sickle Cell Anemia because the body is unable to create new red blood cells quickly enough.
The combination of abnormal cells and anemia resulting in Sickle Cell Disease may result in body tissues being starved of oxygen, causing severe pain and other serious complications including infection, stroke and heart effects.
Oxbryta Treatment for Sickle Cell Disease
Oxbryta (voxelotor) was the first medication approved to treat sickle cell disease and works by increasing the affinity of hemoglobin for oxygen. It was intended to prevent serious complications of sickle cell disease but may increase the risk of a vaso-occlusive crisis and death when compared to placebo. Vaso-occlusive crisis may occur when small blood vessels become blocked and may result in death.
FDA Announces Oxbryta Recall
After the results of two FDA-mandate studies were released, on September 26, 2024, the FDA, along with Pfizer, announced a recall of all lots of Oxbryta. The recall notification states that Oxbryta may cause an increased risk of vaso-occlusive crisis and death which may outweigh any benefits of the medication.
People or loved ones of those who experienced vaso-occlusive crisis, stroke, organ failure or died after taking Oxbryta may be eligible for compensation.